rearden said:
What discharge rates do you consider to be high current?
When does the effect of the Internal Resistance start to become significant?
If we fully charge an empty cell then the reported mAh is very close to its (current)
chemical capacity (assuming that the charge termination current is not set unreasonably high). But the actual
usable capacity that wecan extract from that charge depends on various factors, esp. the discharge rate (current), health (esp. R = total DC IR) and temperature, because - by Ohm's Law - the voltage while (steady-state) discharging is dragged down roughly by I*R at discharge current I, which causes it to sooner reach the termination voltage. If the product I*V is not too large then we'll be able to discharge almost all the charged capacity (typically over 99%), so the charged capacity yields an excellent estimate of the discharge capacity. But this close correspondence breaks down when I*R gets too large. This is easiest to comprehend by looking at
discharge curves at various rates, e.g. below, for two identical NMC Li-ion cells. For each rate there are two similarly colored curves - one for each cell.
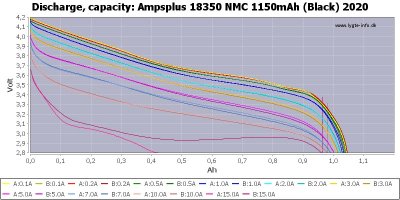
His charge curve shows they charged about 1044mAh, and discharged 1044mAh @100mA and 200mA, and 1039mAh at 500mA, all > 99.45% of the charged capacity. But at higher rates the curves 2.8V termination points move leftward, yielding lower capacity, e.g. at 15A (two lowest curves)the stronger cell yields about 923mAh(92%) and the weaker peters out much earlier at around 416mAh(40%). This is because the I*R drop has essentially pulled down the voltage curve so much that its termination point is now very close to the "knee" of the curve - where it has much smaller slope, so even a small voltage change causes a large capacity change.
To see this more clearly, suppose our termination voltage is 3.0V instead of 2.8V. Then the lowest 2 curves in theabove graph show thatat 15A both cells peter out very early - at around 255mAh(24%) and 310mAh(30%) when they hit 3.0V. As we can see from the stronger cell's curve, most of its energy is delivered in the flat part of the curve below between 3.0V and 2.9V, but we cannot access it because it liesbelow our 3.0V termination voltage. [Note: the reason this curve has flatter shape than the lower rate curves above it is due to self-heating effects. The large 15A rate generates sufficiently more internal heat that it decreases the cells internal resistance I, which decreases the voltage drop I*R. This was enough rise for the stronger cell to deliver most of its energy above 2.8v, but the weaker cell couldn't recover in time].
Those were new cells. If they were more aged theirinternal resistance might be twice as large, so we'd notice the same effects as above at about half the current,since (I/2)(2R) = IR yields the same voltage drop. But stillthe discharge capacity remains above 99% of the charge capacity at the lower rates, so wedon't need to do a discharge to know the (chemical or low-rate)capacity - we can simply use the charged capacity.
NotesFor simplicity I have mostly ignored temperature effects above. But these can play a large role - esp. at the extreme ends of temperature ranges. e.g. in coldwinter termperatures the self-heating effect may make or break whether or not a cell delivers decent capacity (IR
greatly increases at cold termperatures, which is why EV packs often employ heaters).
The linked tests useprofessional equipment.Consumer-level (dis)chargers are less precise and accurate so they may reportslightly more variation between charge and discharge capacitydue to their limitations.